Over the years, working in the business world, particularly in the spine market, I’ve faced my fair share of challenges, and I’ve also seen others navigate their struggles. One thing I’ve observed is how difficult it is for most people to use their intelligence effectively when dealing with frustrations and problems. In today’s article, I want … [Read More...] about Reflections on the Importance of Mastering Emotions: Power with Control!
Main Content
FEATURED ARTICLE
BREAKING NEWS
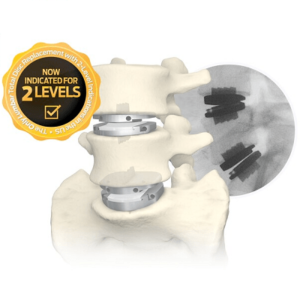
Landmark Lumbar Total Disc Replacement Study of Nearly 1,200 Patients Supports Long-term Clinical Success and Durability of Centinel Spine’s prodisc® L System
WEST CHESTER, Pa., Dec. 12, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced the recent release of a landmark study supporting the … [Read More...] about Landmark Lumbar Total Disc Replacement Study of Nearly 1,200 Patients Supports Long-term Clinical Success and Durability of Centinel Spine’s prodisc® L System
Augmedics Appoints Paul Ziegler as President and Chief Executive Officer
CHICAGO--(BUSINESS WIRE)--Augmedics, a pioneer in augmented reality (AR) surgical navigation, today announced Paul Ziegler as President and Chief Executive Officer. With the appointment, Gwen Watanabe will transition from interim CEO and serve as Vice Chair of the Board. Ziegler, a 20-year medical device veteran, joins Augmedics with a proven track record in commercial and operational … [Read More...] about Augmedics Appoints Paul Ziegler as President and Chief Executive Officer

NGMEDICAL Announces Completion of Patient Enrollment in the one- and two-Level MOVE®-C Clinical Trial
NGMedical announces completion of patient enrollment for its multi-center one- and two-level trial, designed to support a future PMA-application for MOVE®-C. NONNWEILER, GERMANY, December 10, 2024 /EINPresswire.com/ -- NGMedical GmbH, a medical device manufacturer exclusively focused on creating inno-vative technologies for spinal application, announces completion of patient … [Read More...] about NGMEDICAL Announces Completion of Patient Enrollment in the one- and two-Level MOVE®-C Clinical Trial

Countdown to Innovation: INNOVERSE Spinal System Launching December 16th!
We are excited to announce the official launch of the INNOVERSE Spinal System, set for December 16th! This state-of-the-art spinal fixation system is designed to revolutionize spinal surgery with its focus on optimized performance, flexibility, and diversity, ensuring exceptional outcomes for surgeons and patients alike. Optimized Performance The INNOVERSE Spinal System offers unparalleled … [Read More...] about Countdown to Innovation: INNOVERSE Spinal System Launching December 16th!

Atlas Spine Announces FDA Clearance of its HiJAK Expandable Lateral Lumbar Interbody System
JUPITER, FL, December 10, 2024 — Atlas Spine, Inc., a spinal implant company based in Jupiter Florida, announced today they have received 510(k) clearance from the FDA for the HiJAK Expandable Lateral Lumbar Interbody System. Atlas is answering the evolving market needs of lateral fusion procedures with the introduction of HiJAK LLIF. Leveraging … [Read More...] about Atlas Spine Announces FDA Clearance of its HiJAK Expandable Lateral Lumbar Interbody System

Theradaptive Obtains FDA IDE Approval to Study OsteoAdapt™ SP in Additional Spinal Fusion Indications
FREDERICK, Md., Dec. 10, 2024 /PRNewswire/ -- Theradaptive, a privately held, clinical stage biologics company developing protein therapeutics for spine, orthopedics, soft tissue repair, and targeted immuno-oncology, announced today that the US Food and Drug Administration (FDA) has approved an expansion to its Phase I/II clinical program, broadening it to include three spinal fusion … [Read More...] about Theradaptive Obtains FDA IDE Approval to Study OsteoAdapt™ SP in Additional Spinal Fusion Indications

PainTEQ Secures Groundbreaking Patent for LINQ® Technology Advancing Precision and Efficiency in SI Joint Fusion
TAMPA, Fla., Dec. 9, 2024 /PRNewswire/ -- PainTEQ, a leader in minimally invasive sacroiliac (SI) joint dysfunction treatment, has been awarded a new patent (US Patent No. 12,121,458), marking another significant milestone in the advancement of its innovative LinQ® SI Joint Stabilization System. This latest patent expands the breadth of PainTEQ's single-use instrumentation portfolio and protects … [Read More...] about PainTEQ Secures Groundbreaking Patent for LINQ® Technology Advancing Precision and Efficiency in SI Joint Fusion
The Importance of Sales Representatives in the Operating Room: Supporting Spinal Surgeries
In the spine business, certain questions frequently arise: Is the presence of a sales representative in the operating room necessary? Does their involvement increase the company’s risk exposure? While these concerns are valid, the evidence points to a clear conclusion: sales representatives play a critical role in ensuring the success of surgical procedures. Their presence provides surgeons … [Read More...] about The Importance of Sales Representatives in the Operating Room: Supporting Spinal Surgeries
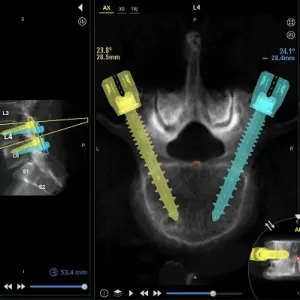
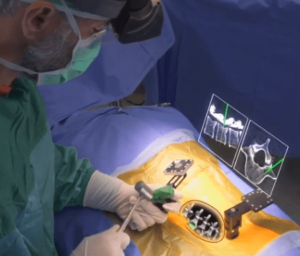
Study at HSS Compares Robotic-Assisted Navigation and Augmented Reality in Spine Surgery
A recent study conducted at the Hospital for Special Surgery (HSS) has shown that both robotic-assisted navigation (RAN) and augmented reality (AR) technologies achieve remarkable precision and safety in placing pedicle screws during spinal surgeries. The findings, published online ahead of print in the journal Spine, represent the first head-to-head comparison of these two advanced surgical … [Read More...] about Study at HSS Compares Robotic-Assisted Navigation and Augmented Reality in Spine Surgery

Curiteva Surpasses 2,000 Procedures With Inspire(R)C Cervical Trabecular PEEK(TM) With HAFUSE(R) Technology
December 4, 2024 (Newswire.com) - Curiteva, Inc., a privately held manufacturing and technology company, today announced it has implanted over 2,000 patients with the Inspire®C Cervical Interbody Fusion Device. This success translates to more than 3,700 implants placed, demonstrating the system's rapid adoption and effectiveness since its commercial launch in July 2023. Chambliss Harrod, M.D., … [Read More...] about Curiteva Surpasses 2,000 Procedures With Inspire(R)C Cervical Trabecular PEEK(TM) With HAFUSE(R) Technology

Tyber Medical Achieves Class III MDR CE Mark Certification from BSI for PEEK Titanium Plasma-Coated Cervical Cages
BETHLEHEM, Pa., Dec. 5, 2024 /PRNewswire/ -- Tyber Medical LLC, a leading provider of private-label orthopedic and spinal implants, proudly announces that it has received Medical Device Regulation (MDR) CE Mark certification from BSI for its PEEK Titanium Plasma-coated cervical cages. This certification for Tyber Medical's innovative technology is a pivotal step toward bringing the … [Read More...] about Tyber Medical Achieves Class III MDR CE Mark Certification from BSI for PEEK Titanium Plasma-Coated Cervical Cages

Introducing the FJ-f Interfacet Implant: Advancing Spinal Stabilization with Innovative Solutions
This year’s EUROSPINE 2024 symposium in Vienna is now behind us. It was a significant event for specialists in spine surgery. The gathering provided an excellent opportunity for showcasing innovations and exchanging experiences among surgeons from various countries. One of the program highlights was the presentation of the FJ-f intra-articular implant (Facet Joint Fusion; patent … [Read More...] about Introducing the FJ-f Interfacet Implant: Advancing Spinal Stabilization with Innovative Solutions

NovApproach Spine Enters International Market
First Surgeries Using OneLIF™ Interbody Fusion Cage in Panama Alachua, Fla. Alachua, Fla. (December 4, 2024) – NovApproach Spine, developer of the patented OneLIF™ interbody spinal fusion system which supports a multitude of surgical approaches, announced today its entry into the Panamanian market with two surgeries performed last week. Dr. Gamaliel Gonzalez Atencio at the Instituto Panameño de … [Read More...] about NovApproach Spine Enters International Market

Carlsmed Announces FDA Clearance for aprevo® Cervical Breakthrough Fusion Device
CARLSBAD, Calif.--(BUSINESS WIRE)--Carlsmed, Inc. (“Carlsmed” or the “Company”) a MedTech company pioneering AI-enabled personalized spine surgery, today announced FDA 510(k) clearance for the aprevo® Cervical ACDF Interbody System. This milestone underscores Carlsmed’s commitment to advancing patient-specific spine surgery solutions that enhance outcomes and surgical precision. The FDA … [Read More...] about Carlsmed Announces FDA Clearance for aprevo® Cervical Breakthrough Fusion Device

A New Era in Back Pain Treatment: Discseel® Procedure is Proven Effective and Able to Replace Most Spine Surgeries in a Recent Groundbreaking Study
DALLAS, Dec. 4, 2024 /PRNewswire/ -- This study is the World's largest Spine Regenerative Medicine study. 3-year results of the Discseel® Procedure were published in Pain Physician Journal and demonstrate high safety and efficacy in treating chronic low back pain and sciatica (radiculopathy.) The study's Lead Investigator, Kevin Pauza MD, Founding Partner of Texas Spine and Joint … [Read More...] about A New Era in Back Pain Treatment: Discseel® Procedure is Proven Effective and Able to Replace Most Spine Surgeries in a Recent Groundbreaking Study

Neo Medical’s Spine Care Platform Fully Approved Under EU Medical Device Regulation Directive
LAUSANNE, Switzerland--(BUSINESS WIRE)--Neo Medical SA (Neo), a Swiss technology company specializing in spine surgery, today announced the approval of its entire product portfolio under the European Union’s (EU) Medical Device Regulation (MDR) EU 2017/745, confirming compliance with the world’s highest quality control standards for medical devices. Certification allows Neo to continue … [Read More...] about Neo Medical’s Spine Care Platform Fully Approved Under EU Medical Device Regulation Directive

Human-Centered Leadership: A Viable Strategy in the Spine Industry?
In the spine implant industry, where innovation directly impacts patient outcomes and precision is non-negotiable, leadership cannot rely solely on traditional approaches focused on managing results. The sector’s complex regulatory environment, intense competition, and constant drive for technological advancements require leaders who go beyond simply overseeing operations—they must inspire, … [Read More...] about Human-Centered Leadership: A Viable Strategy in the Spine Industry?











![[GS Medical] Banner Ad_2-2](https://thespinemarketgroup.com/wp-content/uploads/2024/02/GS-Medical-Banner-Ad-1_ver.2-jpg-750x100.webp)





